Key technologies
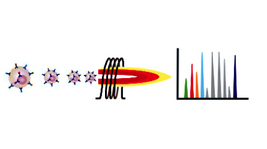
Mass Cytometry
Multiplexed Ion Beam Imaging (MIBI)
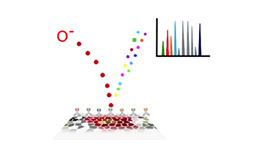
We have developed a method that uses secondary ion mass spectrometry to image antibodies
that are tagged with isotopically pure elemental metal reporters.
Multiplexed ion beam imaging
(MIBI) allows analyzing up to 100 targets simultaneously over a five-log dynamic range in a way
similar to CyTOF, but in addition to measuring protein levels on individual cells, it also provides the
information about cell morphology and localization.
We used MIBI to analyze paraffin-embedded
human breast cancer sections stained with 10 labels simultaneously, providing new insights into
disease pathogenesis that could be valuable for basic research and clinical diagnostics.
Phospho flow

Phospho-specific flow cytometry, or phospho flow, measures the phosphorylation state of
intracellular proteins at the single cell level. This is achieved by a special cell permebealization
strategy and staining intracellular targets with phosphoepitope-specific antibodies.
Many
phosphorylation events can be analyzed simultaneously in each cell, along with cell surface markers,
enabling complex biochemical signaling networks to be resolved in heterogeneous cell populations.
The method has been applied to many diverse areas of biology, including the characterization of
signaling pathways in normal immune responses to antigenic stimulation and microbial challenge,
alteration of signaling networks that occur in cancer and autoimmune diseases, and high-
throughput, high-content drug discovery. We also showed the information about the distribution
of phosphoepitope levels in single cells can be also used to infer the causal structure of intracellular
signalling networks de novo using machine learning methods.

Professor, Microbiology & Immunology - Baxter Laboratory
Member, Bio-X Member, Child Health Research Institute Member, Stanford Cancer Institute Full bio