b-catenin/armadillo (updated September 2008)
b-catenin/armadillo (updated September 2008)
b-catenin (armadillo in Drosophila) is the key mediator of the Wnt signal.
In cells not exposed to the signal, b-catenin levels are kept low through interactions with the protein kinase zw3/GSK-3, CK1a, APC and Axin (Behrens, 1998 Itoh 1998., Hamada, 1999.) b-catenin is degraded, after phosphorylation by GSK-3 and CK1 alpha (Yanagawa 2002, Liu 2002, Amit 2002), through the ubiquitin pathway (Aberle 1997.), involving interactions with Slimb/bTrCP (Jiang 1998, Marikawa 1998,; reviewed in Maniatis 1999)
In a current model, Wnt signaling initially leads to a complex between Dsh, GBP/Frat1, Axin and Zw3/GSK, which may be the regulatory step in the inactivation of Zw3/GSK (Salic, 2000; Farr 2000). The DIX domain in Axin is similar to the NH2 terminus in Dsh, and promotes interactions between Dsh and Axin (Hsu 1999, Smalley, 1999). As a consequence, GSK does not phosphorylate b-catenin anymore, releasing it from the Axin complex and accumulation (Salic, 2000).The stabilized b-catenin then enters the nucleus to interact with TCF. b-catenin can convert TCF into a transcriptional activator of the same genes that are repressed by TCF alone (reviewed in Nusse, 1999). Two other key players in this complex are Legless (Bcl9) and Pygopos (Kramps 2002, Thompson 2002, Parker 2002). In mammalian cells and in the Zebrafish, Bcl9-2 regulates binding of b-catenin to the adhesion complex or its presence in the nucleus, by interacting with the tyrosine phosphorylated form of b-catenin (Brembeck, 2004).
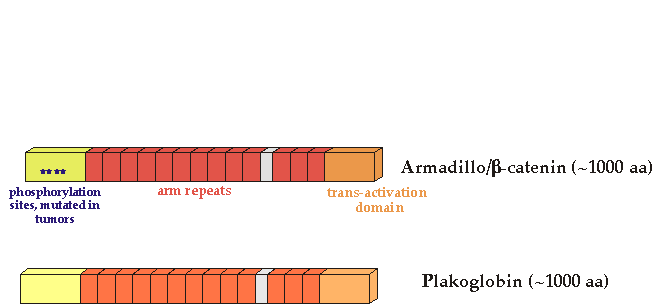
Activating mutations in the human b-catenin gene have been found in human colon cancer and melanomas (Morin et al, 1997) These mutations alter specific b-catenin residues important for GSK3 phosphorylation and stability. There is a separate list of mutations.
Vertebrates have two Armadillo/b-catenin homologs, b-catenin and plakoglobin (also called gamma-catenin). It is not clear whether plakoglobin is mutated in cancer, although overexpression of mutant forms can transform cells (Kolligs, 2000) . Both plakoglobin and b-catenin bind to cadherins to establish cell adhesion.
There are three b-catenin genes in C. elegans. Interestingly, one of them (HMP-2) is dedicated to adhesion only, whereas BAR-1 and WRM-1 act in Wnt signaling(Korswagen 2000 Natarajan 2001) Schneider et al (2003) postulate that a single, b-catenin gene fulfilled both adhesion and signaling functions in the last common ancestor of metazoans some 700 million years ago.
See table C. elegans Wnt signaling.
| Human | Mouse | Mouse phenotype | C. elegans | Drosophila |
|---|---|---|---|---|
| b-catenin | b-catenin | Many including
|
BAR-1 | armadillo |
| . | . | . | WRM-1 | . |
| plakoglobin | plakoglobin | . | HMP-2 | . |